It’s time to talk about ‘fats’.
I’m not talking about the stuff under your skin and jiggles when you jump, but the kind that you eat in food like drizzle on your salad or spread on your toast (actually, they are kinda the same thing).
Everyone’s got an opinion about them and unfortunately, most people only know about the misconceptions of fats. There are many types of fat and so when you are talking about fats, you should know what type you’re referring to. It is important to note, however, that all forms of fat that you eat actually consists of a combination of all of them! It’s only a matter of what the item has more of!
I’m going to try to walk the fine line between giving a detailed explanation so you understand the fundamentals of fats without boring you with all the science and losing your attention. Wish me luck!
If, however, you just want to skip to the summary, then you can skip all the explanation and click here.
The Lipid Hypothesis
The story begins back in the late 1950’s when a researcher named Ancel Keys proposed what is now known as the Lipid Hypothesis which claims that high saturated fat and cholesterol in your diet leads to heart disease.
However, there were many other researchers that challenged his claim and provided many scientific studies to the contrary but the public media had already by that time pushed across the United States; and not too long thereafter, the rest of the world that eating too much saturated fats was going to clog your arteries and give you heart attacks.
Let’s come back to this story after a little bit of science now.
The Chemistry of Fats (or Lipids)
There are lots of different types of fats and as you probably already know, some are healthier than others. Some are used for machinery to make them operate smoothly while others are used in your body to keep you living. They are all called fats, fatty acids, oils or lipids – they are all synonyms and I will be using them interchangeably through out this blog.
The chemical structure of all fatty acids is a chain of carbon atoms with hydrogen atoms connected to the top and/or bottom of each carbon atom, with a head (a glycerol molecule) and a tail. Don’t worry about the head or tail as that’s not as important as the carbon chain and hydrogen atoms.
There are 2 main ways of classifying fatty acids:
- classification by length
- short-chain
- medium-chain
- long-chain
- classification by saturation
- saturated fats
- unsaturated fats
- monounsaturated fats
- polyunsaturated fats
The length of fatty acid although is important, is not that interesting for there are health and unhealthy fats for each lengths so going into the details in this classification won’t be really that useful to you (and will probably bore you).
The classification by saturation, however, is why you are all here for as you are dying to know if saturated fats are really as evil as everyone says they are. So let’s dive in to the details.
What does ‘Saturated Fats’ mean?
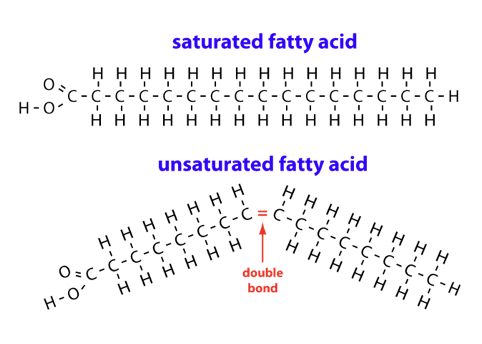
As mentioned above, the carbon atoms have hydrogen atoms connected to them. Imagine a chain of carbon atoms linked together one after another like a train.
H is for hydrogen atom
C is for carbon atom
In Figure 1 above, you can see under “saturated fatty acid” (SFA) that all the carbon atoms in the chain have a hydrogen atom linked to either side of them.
Whereas, in the “unsaturated fatty acid” chain has a pair of carbon atoms that have 1 missing hydrogen atom each. There is a kink where the 2 missing hydrogen atoms are and forces the carbon chain to bend at that point. This bendy joint is called a ‘double bond’ in chemical terms and is an important point that I will come back to later.
Since the SFA chain is completely filled (or saturated) with hydrogen atoms, its chemical structure is straight and hence, stack up neatly and steadily together. This is why saturated fats are solid in room temperature.
Examples of food that are high in SFA are butter, lard (pork fat), beef tallow, duck and fat, coconut oil, palm oil.
When unsaturated fatty acids have 1 bend in them, they are called monounsaturated fats (MUFA).
Examples of food high in MUFAs are olive oil, peanut oil, sesame oil, avocado oil, and nuts like cashews, pecans, almonds, hazelnut, and canola.
When unsaturated fatty acids have multiple bends in them, they are called polyunsaturated fats (PUFA).
Examples of food high in PUFAs are fish oils, walnut oils, soybean oil, seed oils like sunflower, safflower, cottonseed, grapeseed, flaxseed and chia.
Since MUFAs don’t stack as neatly as SFAs in room temperature, they are usually liquid and will solidify in the refrigerator.
PUFAs having even more kinks will pack up even more irregular thus will be liquid in room temperature and even if put in the refrigerator.
This is a good and accurate way to know what kind of fat you are dealing with. Remember, all fats we eat contain all the classifications of fats mentioned above, just in different ratios.
Ok, that’s a lot for one week. I will continue going deeper into the science and nutrition of fats in next week’s blog!
In summary,
- There are tons of misconceptions on fat – so don’t trust everyone who gives you nutritional advice on them! Educate yourself (by reading and re-reading this blog)!
- The Lipid Hypothesis was proposed by Ancel Keys back in the 1950s and claimed saturated fats and cholesterol leads to heart disease – we will come back to this claim.
- All the fat in food are made up of all the different classes of fat, just in different proportions. They are not just of one type.
- Fats are classified by how long the fatty acid chain is, and by their saturation.
- Saturated fats are full of hydrogen atoms and are solid in room temperature – think of animal fats.
- Unsaturated fats are usually liquid in room temperature and have kinks in their chains.
Stay tuned as this information could change the rest of your nutrition life once you open Pandora’s box of fat!
We’re doing all this for a higher quality of life!